Abstract
BackgroundFLT3 inhibitors (FLT3i) represent an important new salvage option in patients with relapsed FLT3 mutated AML but response rates are low and survival outcomes remain poor. Many patients with FLT3 AML have co-occurring mutations suitable for sequential molecular monitoring by RT-qPCR, which can be used to identify impending haematological relapse (Heuser, Blood 2021; Ommen, Blood 2010). However, treatment strategies for molecular relapse are not well defined and many patients receive multi-agent chemotherapy or proceed directly to allogeneic stem cell transplant (allo-SCT) despite evidence of poor outcomes (Hourigan, JCO 2019; Dillon, Blood 2020). We aimed to describe response rates and outcomes in patients treated with FLT3 inhibitors for molecular persistence, progression or relapse (collectively 'molecular failure').
Methods In the UK, molecular monitoring for AML is largely centralised, and intervention with FLT3i monotherapy at the time of molecular failure is suggested for patients who had a FLT3 mutation at diagnosis. Consecutive patients undergoing FLT3i salvage were retrospectively identified from 27 participating hospitals. Patients met ELN criteria for molecular failure: persistence >2% after chemotherapy, relapse after previously negative results or progression of ≥1 log10 from low-level positivity.
Response was assessed by sequential MRD measurement and was censored at the time of allo-SCT, donor lymphocyte infusion (DLI) or subsequent chemotherapy. Molecular response (MR) was defined as a ≥1 log10 reduction from the pre-treatment result. CRMRD-, overall (OS) and event-free survival (EFS) were defined as per ELN. Molecular event-free survival (mEFS) included molecular progression or relapse, haematological relapse or death. Stored pre and post-treatment samples were retrospectively analysed using a sensitive NGS-based FLT3-MRD assay (Blatte, Leukemia 2019).
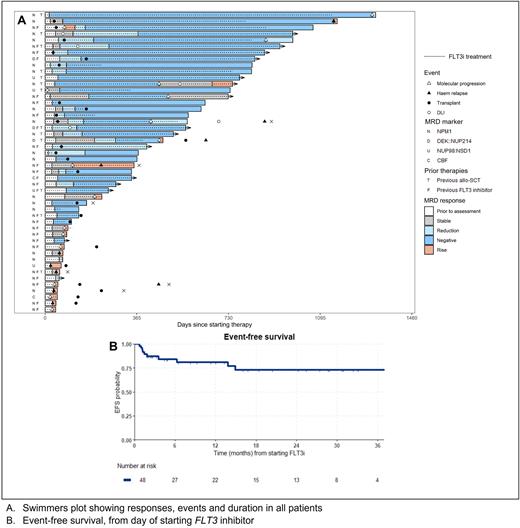
Results Forty eight patients were identified, aged 5 to 76 years. Thirty-nine (81%) had NPM1 mutations, 4 NUP98::NSD1 fusion, 3 DEK::NUP214, and 1 each CBFB::MYH11 and RUNX1::RUNX1T1. At diagnosis, 44 (92%) had a FLT3-ITD, 6 FLT3-TKD and 2 had both. Molecular failure occurred at a median of 9.6 months (range 0.9 - 31) from AML diagnosis and comprised molecular persistence in 6 (12%), molecular progression in 19 (40%) and molecular relapse in 23 (48%). Patients had received a median of 1 prior line of therapy (range 1-3), which included a FLT3i in 25 (52%) and allo-SCT in 13 (27%).
Thirty-two (67%) patients were treated with gilteritinib while 8 (17%) received quizartinib and 8 (17%) sorafenib. Data on treatment toxicity in the first 4 cycles was available for 21 patients. The median number of days of neutropenia and thrombocytopenia and median number of transfusions per cycle was 0. Only 6 of the 21 patients required hospitalisation at any time in the first 4 cycles.
Response was assessable in 47 patients, with 30 (64%) achieving MR of whom 19 (40%) had CRMRD-. Six patients (13%) had stable disease and 11 (23%) progressed, including 5 with haematological relapse. Patients treated for molecular relapse had the highest rate of CRMRD- at 55%. CRMRD- was lower in patients with prior FLT3i but the rate of MR was similar, apart from patients currently receiving FLT3i at the time of molecular failure of whom 6 of 13 (46%) had progressive disease. Patients with prior allo-SCT had a high response rate, with 92% achieving MR. Stored pre-treatment samples were available for FLT3 NGS-MRD analysis in 24 patients, with an ITD detected in 20 at a median VAF of 0.04%. The response rate in these patients was 70%.
Twenty-four patients received allo-SCT after FLT3i salvage, 18 without requiring additional therapy prior to transplant. Seven patients received DLI. Responses deepened after allo-SCT or DLI, with 77% achieving CRMRD-.
At 24 months, OS was 80% (95% confidence interval [CI] 67-96), EFS was 73% (95%CI 59-90) and mEFS was 53% (95%CI 39-72). Late relapses after stopping therapy were noted in 4 patients.
Conclusions In contrast with FLT3i monotherapy at the time of frank relapse, treatment at molecular failure is associated with low toxicity, high rates of molecular response and encouraging overall survival, with many patients able to be bridged to allo-SCT or DLI. These results suggest that in patients with an appropriate marker, sequential molecular MRD monitoring should be utilised and the results used to guide pre-emptive therapy
Disclosures
Khan:Astellas: Speakers Bureau; TC BioPharm: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria. Krishnamurthy:Astellas: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Latif:Abbvie: Honoraria; Kite: Honoraria; Novartis: Honoraria; Jazz: Honoraria; Takeda UK: Honoraria; Astellas: Honoraria. Belsham:Celgene: Other: Meeting sponsorship; Abbvie: Other: Meeting sponsorship. Conneally:Jazz: Honoraria; Novartis: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria. Craddock:Abbvie: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy. Culligan:Abbvie: Honoraria, Other: Meeting sponsorship; Celgene/BMS: Honoraria, Other: Meeting sponsorship; Gilead: Honoraria; Jazz: Honoraria; Takeda: Honoraria. Ingram:Gilead: Consultancy; Novartis: Consultancy, Honoraria; Takeda Oncology: Honoraria, Speakers Bureau; SOBI: Consultancy. King:Astellas: Honoraria. McMullin:Incyte: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; AOP: Research Funding, Speakers Bureau; CTI: Consultancy; Sierra Oncology: Consultancy; BMS: Consultancy, Research Funding; Pfizer: Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Russell:Servier: Honoraria; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau; Astellas: Honoraria. Dillon:AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen Inc.: Research Funding; Astellas Pharma Inc.: Consultancy, Honoraria, Speakers Bureau; AvenCell Europe GmbH: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis AG: Consultancy, Honoraria, Speakers Bureau; Pfizer Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck Labs: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
FLT3 inhibitors are FDA approved in specific clinical scenarios. This presentation will discuss their use as MRD-guided therapies which is not an FDA approved indication
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal